Recently, Professor Li Aifeng from College of Environmental Science and Engineering, in collaboration with Professor Lin Senjie’s team from Xiamen University, has made significant progress in the field of the biosynthesis mechanism of β-N-methylamino-L-alanine (BMAA) in diatom cells. Their findings, titled “Induction of a neurotoxin in diatoms by iron limitation via cysteine synthase”, have been published online in the top-tier multi-disciplinary journal PNAS on June 9.
In this study, the researchers selected diatom strains such as Thalassiosira minima and Phaeodactylum tricornutum, and revealed for the first time the biosynthetic pathway of protein-bound BMAA in diatoms through transcriptomic analysis, prokaryotic expression and purification of the target gene, in vitro biochemical assays, and CRISPR/Cas9 gene knockout techniques. This breakthrough lays a solid foundation for further exploration of the biosynthesis and environmental regulation mechanisms of BMAA toxins in marine diatoms.
The study found that all 12 diatom strains produced protein-bound BMAA, but no free form was detected. This finding indicates that the BMAA toxin synthesized by diatoms in indoor culture is primarily in the protein-bound form, i.e., BMAA is incorporated into proteins. Under Fe-limited conditions (1/3 × Fe), all the 12 diatom strains exhibited growth suppression. However, BMAA synthesis increased by 7.47-folds in eight of the 12 BMAA-producing species, with the most notable increase observed in Thalassiosira minima. This suggests that it is prevalent that Fe limitation induces BMAA synthesis in diatoms.
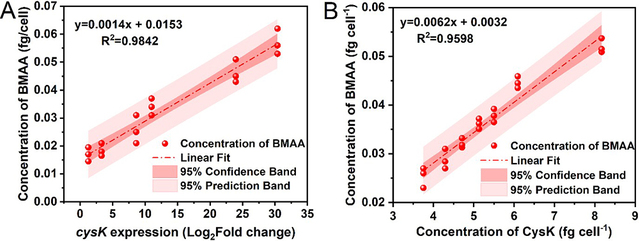
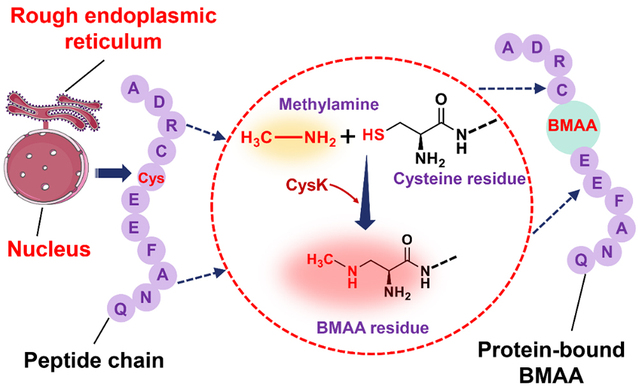
Transcriptome analysis gradually revealed the regulatory role of cysteine CysK in BMAA synthesis in diatoms. KEGG enrichment analysis suggested that the synthesis and accumulation of BMAA-containing proteins might be related to intracellular protein synthesis and post-translational modifications. Based on previous transcriptomic analysis, the study found that the significant upregulation of the CysK gene correlates with increased BMAA synthesis (Fig. 1). It is therefore hypothesized that CysK may act as the core catalytic enzyme in the biosynthesis of BMAA, mediating the process of its generation.
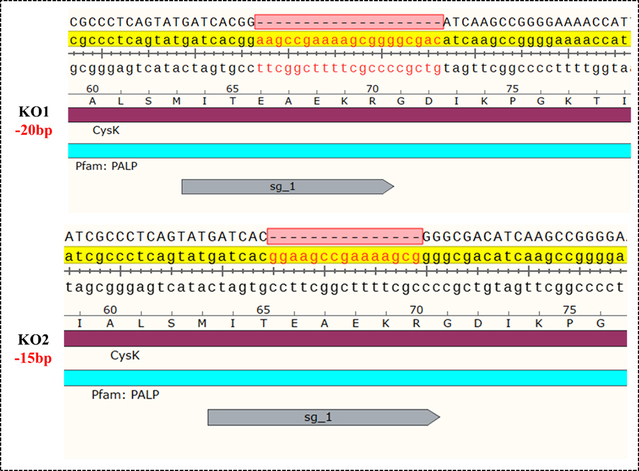
The research identified the role of CysK in BMAA biosynthesis through prokaryotic expression and in vitro enzyme activity assays. The addition of exogenous CysK and methylamine was found to promote the production of BMAA in diatom cells. Using CRISPR/Cas9 technology, the cysK gene was knocked out in diatom cells, resulting in two gene-edited strains (Fig. 2). Analysis showed that both strains lost the ability to synthesize BMAA. After the exogenous addition of prokaryotically expressed and purified CysK protein, the gene-edited diatoms regained the ability to produce BMAA, further confirming the critical role of CysK in the biosynthesis of the BMAA toxin in diatom cells.
This study reveals the molecular mechanisms underlying BMAA synthesis in typical marine diatoms, and clarifies the catalytic role of Cysk and its regulatory network under Fe limitation. It provides a novel perspective on the synthesis of marine phytoplankton toxins, which is of great significance for protecting marine ecosystems and human health.