Recently, Professor Li Ming from the School of Medicine and Pharmacy, in collaboration with Professor Li Xuechen from the University of Hong Kong and Professor Chen Sheng from the Hong Kong Polytechnic University, has made significant progress in the synthesis of capsular oligosaccharides from Campylobacter jejuni and the evaluation of their immunogenicity. Their research findings, titled “Synthesis of Campylobacter jejuni Capsular Oligosaccharides and Identification of a Potential O-Antigen against Campylobacteriosis,” have been published in the internationally renowned journal Science Advances.
The capsular polysaccharide of Campylobacter jejuni features a unique and rare heptose structure, the key virulence factor for the bacterium's infection. It can also be developed and applied as a potential antigen for novel antibacterial glyco-conjugate vaccines. According to statistics, the serotype HS:4 is the most common strain causing Campylobacter jejuni infections. The research team targeted the capsular polysaccharide derived from Campylobacter jejuni CG8486 (HS:4) to conduct precise synthesis of the capsular oligosaccharides and evaluate their immunogenicity.
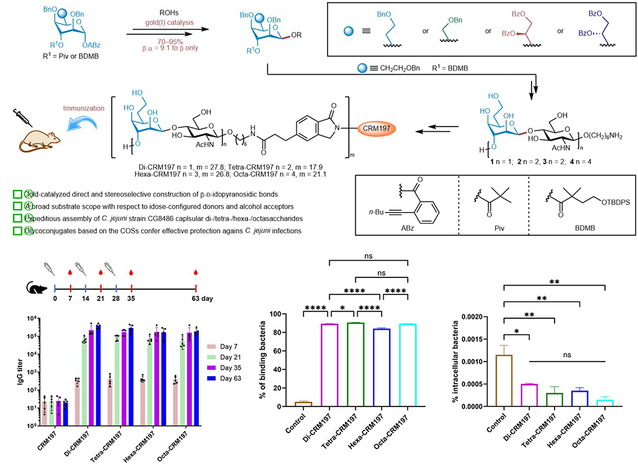
In 2021, the research team successfully completed the total synthesis of the hexasaccharide from Campylobacter jejuni CG8486 for the first time (J. Am. Chem. Soc. 2021, 143, 11171-11179). Following this significant achievement, the team developed a universal and precise synthesis strategy for the β-D-Aldose glycosidic bond using gold(I)-catalyzed ortho-alkynyl benzoate glycosylation reactions. This strategy is applicable to a variety of alcohol acceptors and four types of Aldose donors (α-D-6-deoxy-aldopentose, α-D-aldose, and D-/L-glycerol-type α-D-aldopentose), enabling stereoselective β-glycosylation reactions. Ultimately, the team achieved multi-gram scale preparation of the key capsular disaccharide repeating unit from the CG8486 strain, as well as efficient assembly of disaccharides, tetrasaccharides, hexasaccharides, and octasaccharides. The researchers precisely synthesized four types of glycoprotein complexes (Di-CRM197, Tetra-CRM197, Hexa-CRM197, and Octa-CRM197), and conducted a systematic immunological evaluation. The results indicated that the glycoprotein complex Di-CRM197 exhibited similar immunogenicity to other protein complexes, suggesting that the structurally simplest disaccharide could serve as the minimal antigenic epitope for the development of vaccines against Campylobacter jejuni. This study not only established a universal method for the efficient synthesis of oligosaccharides containing β-D-Aldose units, but also laid a solid foundation for the development of glyco-conjugate vaccines to prevent Campylobacter jejuni infections.