Recently, Professor Wang Guoqing from the Institute of Marine Biodiversity and Evolution, in collaboration with Professor Lin Hong's team from the College of Food Science and Engineering, has made new breakthroughs in the research on aquatic microbial pathogen control. Their research outcomes, titled Nanodot-Inspired Precise Bacterial Gene Suppression in a Smart Hydrogel Bandage for Underwater Wound Healing and Streamlining Bacterial Gene Regulation via Nucleic Acid Delivery with Gold Nanoclusters, have been published in Advanced Science and Small.
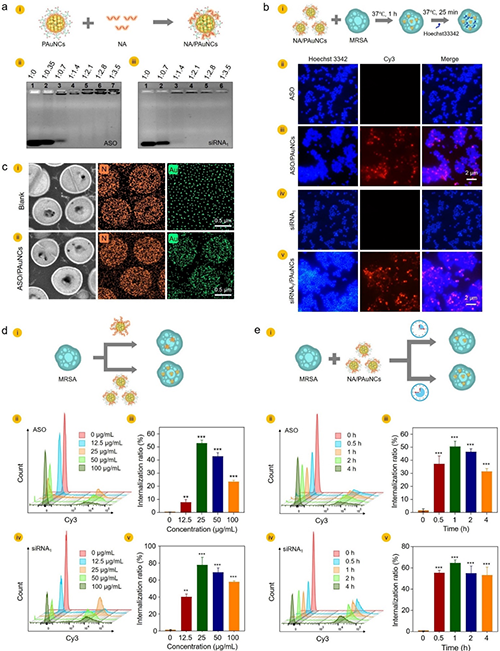
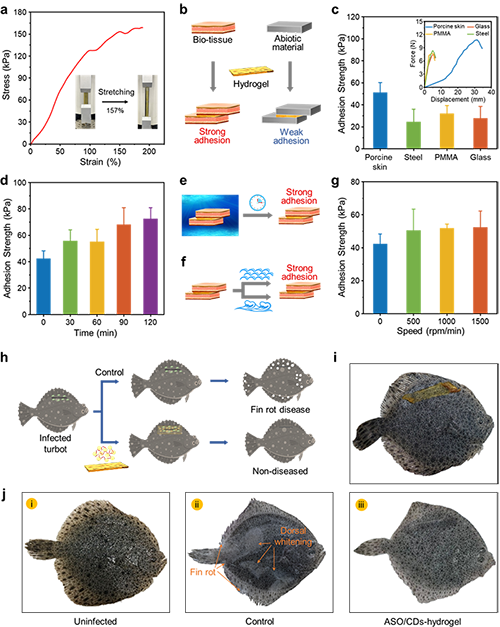
In recent years, high-density aquaculture and environmental pollution have created favorable conditions for the outbreak of Vibrio cholerae. As a pillar industry of marine aquaculture in northern regions, turbot farming has been threatened by diseases caused by marine Vibrio pathogens, such as Vibrio anguillarum. Once these pathogens spread, the mortality rate of turbots would reach as high as 80% within just a few days, resulting in significant economic losses. Nucleic acids like antisense oligonucleotides (ASO) and small interfering RNA (siRNA) can specifically bind to target messenger RNA (mRNA). This binding facilitates the degradation of the mRNA and inhibits protein translation, thereby suppressing target gene expression. However, ASO and siRNA cannot penetrate the bacterial cell membrane to exert their gene regulation functions, limiting their application in the control of aquatic microbial pathogens. Nanostructures with unique size and surface characteristics have been widely used as next-generation delivery materials for nucleic acid delivery to eukaryotic cells. But how to utilize these nanostructures for delivering nucleic acids to bacteria remains a significant challenge.
By utilizing biocompatible nanoscale materials such as carbon dots (CDs) and gold nanoclusters (AuNCs), researchers efficiently delivered siRNA and antisense oligonucleotides (ASO) into pathogenic bacteria like Vibrio anguillarum, thereby inhibiting the expression of specific target genes. Their experiments not only unveil the processes, patterns, mechanisms, and release principles involved in delivering ASO to the bacterial cells, but also integrate this technology with underwater adhesive hydrogels to create a Hydrogel Bandage. This innovative approach has enhanced the targeted efficacy of wound healing in infected tissues, and offered a practical solution for preventing and controlling bacterial diseases in aquatic environments.