Recently, the team led by Academician Xue Changhu from the College of Food Science and Engineering has achieved new research progress in cellular agriculture. Their research findings, titled Scalable production of muscle and adipose cell-laden microtissues using edible macroporous microcarriers for 3D printing of cultured fish fillets, were published in Nature Communications.
As global population growth intensifies pressure on resources and the environment, cellular agriculture has emerged as a prominent research area in the food sector due to its potential in sustainable food production. By using cell cultivation techniques to produce meat, cellular agriculture can reduce the environmental impact of traditional animal husbandry and fisheries, while also improving food safety and animal welfare. However, achieving large-scale cell proliferation and accurately mimicking the characteristics of natural meat remain key challenges for the industrialization of cultured fish fillets.
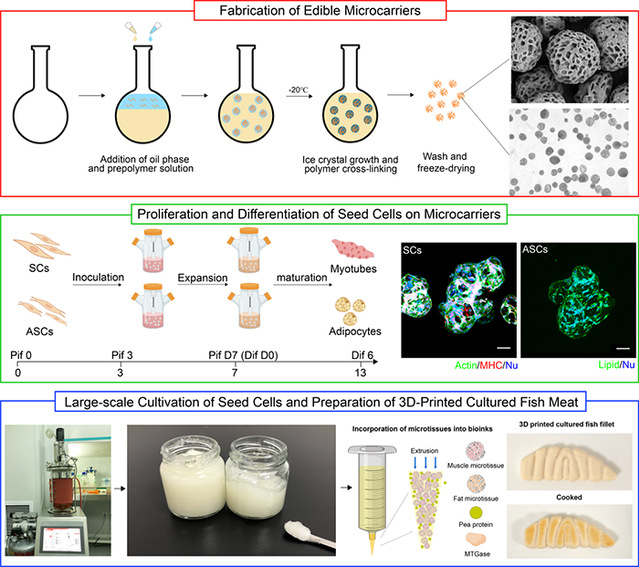
This study creatively developed a cell culture technique based on edible macroporous microcarriers (EPMs) (Figure 1), and successfully designed the microcarriers' macroporous structure through precise control of ice crystal growth. Edible macroporous microcarriers (EPMs) were prepared using a low-temperature emulsification crosslinking technique with fish gelatin and microbial transglutaminase (mTG) as primary materials. These EPMs enabled efficient adhesion, proliferation, and differentiation of large yellow croaker muscle satellite cells (SCs) and adipose-derived stem cells (ASCs). Through precise control of the microcarrier pore size, the impact of the EPMs' porous structure on cell proliferation was further studied. The results indicate that macroporous EPMs provide a favorable growth environment for cells, significantly improving cell adhesion and growth efficiency, and facilitating the expression of genes related to stem cell cycle, cell differentiation and extracellular matrix remodeling.
By utilizing EPMs, the team further developed a gradient scale-up technique for stem cell culture. Following successive expansion, SC and ASC densities reached 6.25 × 10⁵ cells/mL and 5.77 × 10⁵ cells/mL, respectively, representing a 499-fold and 461-fold increase in cell numbers. Furthermore, the researchers integrated mature cell-laden microtissues into bioinks, and used 3D printing to produce cultured fish fillets that not only exhibit sensory characteristics similar to natural fish fillets but also achieve a uniform distribution of muscle and fat cells, mimicking the texture and nutritional components of their natural counterparts. This research overcomes key bottlenecks in efficient cell expansion and food texturization for cultured fish fillets, providing a theoretical foundation and technical support for the sustainable production of these fillets and bolstering the development of future food.