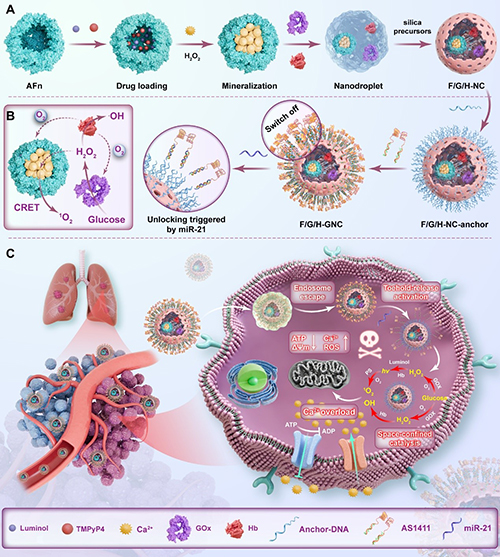
The research team led by Prof. Jiang Shuai from the School of Medicine and Pharmacy, and Prof. Mao Xiangzhao from the College of Food Science and Engineering at Ocean University of China, in collaboration with Prof. Katharina Landfester from the Max Planck Institute for Polymer Research, has developed a novel enzyme-catalyzed anti-tumor nanodrug. Their research findings titled Self-Sustained Biophotocatalytic Nano-Organelle Reactors with Programmable DNA Switches for Combating Tumor Metastasis were published in Advanced Materials, a top-tier international journal in nanotechnology.
Tumor metastasis remains the primary cause of cancer-related mortality. Despite extensive research, effective therapeutic strategies against tumor metastasis have yet to achieve significant breakthroughs. Photodynamic therapy (PDT) has demonstrated great potential in various cancer treatments due to its spatiotemporal activation, non-invasiveness, and low toxicity. However, the clinical application of PDT is confronted with notable limitations in treating deep metastatic lesions owing to limited light penetration depth. Moreover, oxygen consumption during PDT exacerbates the hypoxic tumor microenvironment, leading to upregulated hypoxia-inducible factor-1α (HIF-1α) expression that aggravates tumor invasion and metastasis.
To overcome these limitations, the researchers developed a DNA-gated bionic nano-reactor for efficient and specific catalytic therapy against tumor metastasis. This nano-reactor utilizes glucose as a biofuel to generate H2O2, which subsequently reacts with the high-energy luminescent molecule luminol to generate intense chemiluminescence, enabling PDT driven by chemical energy. The co-encapsulated hemoglobin not only supplies oxygen for both glucose bioconversion and PDT, but also exhibits peroxidase-like activity to amplify the generation of reactive oxygen species (ROS). Crucially, the nano-reactor's catalytic function can be deactivated through DNA switches and specifically re-activated in tumor tissues via nucleic acid strand displacement reactions, ensuring targeted therapy while minimizing off-target effects. Both in vitro and in vivo experiments found that this self-sustained photodynamic system achieves self-sufficiency of light and oxygen and precise tumor targeting, offering a promising solution to treat highly metastatic cancers. This cell-inspired compartmentalized encapsulation strategy enables flexible integration and synergistic delivery of multiple therapeutic modules (including small molecules, proteins and nucleic acids), significantly expanding its potential applications in tumor metabolic therapy, chemiluminescence imaging and synergistic gene therapy.