Recently, the team led by Prof. Li Dehai from OUC’s School of Medicine and Pharmacy, the MoE Key Laboratory of Marine Drugs, the Marine Drugs and Biological Products Function Laboratory of Qingdao Marine Science and Technology Center, and the Marine Biomedical Research Institute of Qingdao, has made significant advances in marine-derived NLRP3 inflammasome inhibitors. The research findings have been published in the prestigious journals Journal of the American Chemical Society and Angewandte Chemie International Edition. The papers are titled “Sorbremnoids A and B: NLRP3 Inflammasome Inhibitors Discovered from Spatially Restricted Crosstalk of Biosynthetic Pathways” and “Discovery, Total Synthesis, and Anti-inflammatory Evaluation of Naturally Occurring Naphthopyrone-Macrolide Hybrids as Potent NLRP3 Inflammasome Inhibitors”, respectively.
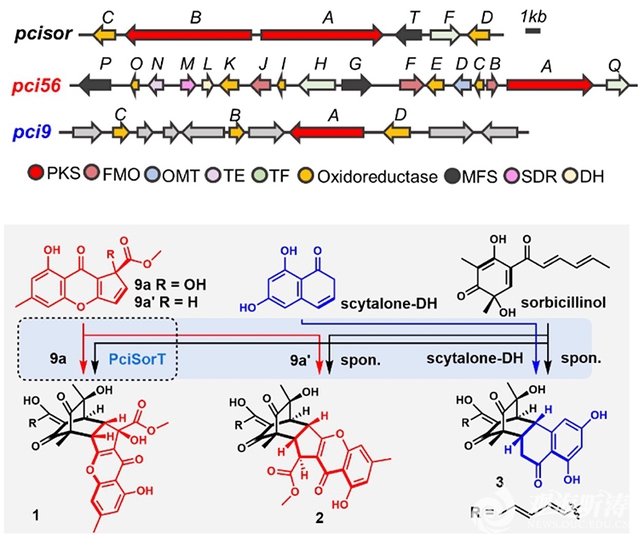
The researchers discovered three hybrid natural products (hbNPs) with novel skeletons—Sorbremnoid A (1), Sorbremnoid B (2), and Sorbtalone (3)—by activating the silent biosynthetic gene clusters in the endophytic fungus Penicillium citrinum HDN11-186, isolated from the Suaeda salsa growing in coastal salt-flats. During the biosynthesis of compounds 1-3, three independent gene clusters (pci9, pci56, and pcisor) synthesized highly reactive monomers (9a/b, scytalone-DH, and sorbicillinol) and formed hybrid dimers through regio- and stereoselective IMDA cyclization. Using gene knockout, quantum chemical calculations, and fluorescence-labeled protein localization, the researchers proposed the spatial biosynthesis process of these dimers at the cellular level. They found that Sorbremnoid A directly targets the NLRP3 protein, effectively inhibiting the assembly and activation of the NLRP3 inflammasome, and promoting healing of hard-to-heal wounds in diabetic mouse models.
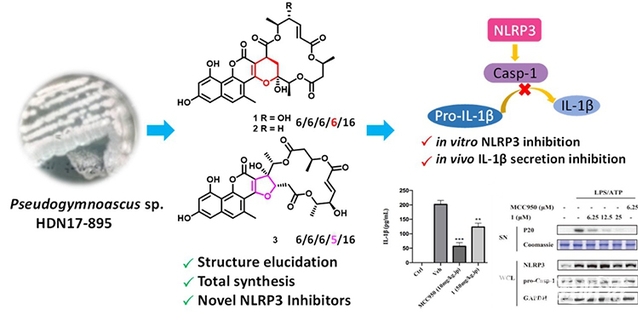
Furthermore, the team isolated a series of unique polycyclic skeleton naphthopyrone-macrolide hybrids, gymnoasins A-C, from the metabolic products of the fungus Pseudogymnoascus sp., collected from intertidal sediment samples from the Fildes Peninsula in Antarctica. Using the Michael addition/hemiketalization cascade reaction, they achieved the total chemical synthesis of gymnoasin A, accurately identifying its chemical structure and addressing the issue of natural drug supply constraints. Subsequent studies revealed that gymnoasin A exhibits anti-inflammatory effects by specifically inhibiting the NLRP3 inflammasome in both cellular and animal models.