On January 7, 2026, Professor He Yan from Professor Zhang Quanqi’s research team at the College of Marine Life Sciences, Ocean University of China (OUC), published a research article entitled “Viviparity and Beyond: Captured Endogenous Retroviral Envelope Genes Drive Teleost Physiological Innovations” in Molecular Biology and Evolution, a leading journal in evolutionary biology. Using the viviparous teleost Sebastes schlegelii as a model, the study not only pinpoints candidate viral genes that drive the origin of viviparity in teleosts but also unexpectedly reveals that these viral genes, “domesticated” by the host, have functions beyond placental development: they are deeply involved in the development of the nervous system and the regulation of male reproduction. This work shows that endogenous retroviral envelope (ERV env) genes act as an “engine of evolution”, playing multifaceted roles in the evolution of complex traits in vertebrates.
In S. schlegelii, the team identified Seb-env3, a class of ERV env genes that are restricted to species of the genus Sebastes and have been conserved for around 15 million years under purifying selection. Seb-env3 fulfils the defining criteria of a syncytin: its transcripts are highly and specifically expressed at the maternofetal interface of the ovarian follicular placenta (granulosa cell layer), and the encoded protein retains strong fusogenic activity. In addition, Seb-env3 contributes to the formation of key structures of the follicular placenta—the microvilli and vesicles—that facilitate nutrient transfer from the mother to the embryo. These findings demonstrate that the hypothesis of viral env genes driving the evolution of viviparity also holds for teleosts, providing an important piece of the puzzle in understanding placental evolution in non-mammal vertebrates.
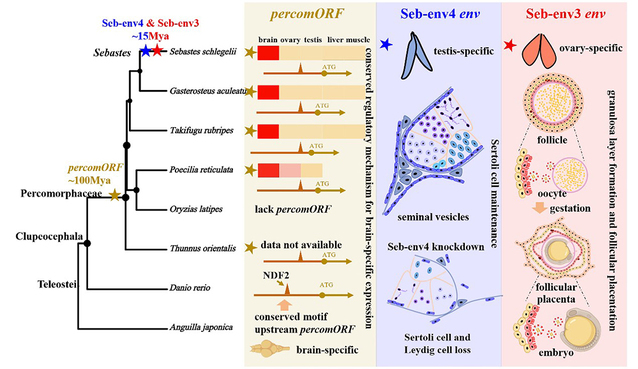
In the course of searching for “fish syncytins”, the research team unexpectedly identified two additional env genes with distinct functions, revealing multidimensional innovation in viral gene co-option. One is percomORF, an ancient env gene that is highly conserved across Percomorpha and shows brain-specific high expression, with its transcription tightly regulated by NDF2, the host neurogenic differentiation factor. The capture of this gene predates the origin of viviparity in the genus Sebastes, suggesting that it may have been co-opted by ancestral Percomorpha to contribute to the formation or signaling of neural circuits. The other, Seb-env4, is specific to S. schlegelii and has been integrated into the regulatory mechanisms of the male reproductive system.
This study reveals that ERVs are not only “architects” of the origin of viviparity in fish, but also “versatile drivers” of physiological innovation in vertebrates. From the ancient nervous system (Percom-env), to more recent maintenance of male reproduction (Seb-env4), and to the key step underpinning the evolution of viviparity (Seb-env3), the findings vividly illustrate how host genomes have repeatedly and independently captured viral genes and repurposed them as engines for the evolution of complex physiological functions.
Over the years, Professors He Yan and Zhang Quanqi and their team have carried out systematic research on genetic breeding and the adaptive evolution of viviparity in S. schlegelii. They have generated a chromosome-level genome assembly for this species (Molecular Ecology Resources, 2019), elucidated its molecular mechanism of sex determination (Open Biology, 2021), and systematically dissected the molecular basis of its viviparity-related adaptive traits (Development, 2024a, 2024b; iScience, 2024; Communications Biology, 2025). The findings now reported in Molecular Biology and Evolution represent an important advance in the team’s research on the origin and evolution of viviparity in fishes.







