Recently, Professor Jiang Tao from the School of Medicine and Pharmacy at Ocean University of China (OUC), together with Professor Li Xuechen from the University of Hong Kong, published online a research paper entitled “Achieving cysteine-selective peptide/protein bioconjugation via tunable triazine-pyridine chemistry” in Science Advances.
Chemical labeling of proteins and peptides is a key technique and fundamental science issue for elucidating protein and peptide functions, developing antibody-drug conjugates (ADC) and peptide-drug conjugates (PDC), and designing targeted therapeutics. The site-specific introduction of chemical modifications or functional groups into peptides or proteins not only provides powerful tools for revealing the molecular mechanisms of life processes but also facilitates the translation of basic research into practical applications. From clarifying the dynamic relationships between protein structure and function to developing highly effective targeted antibody-drug conjugates, selective bioconjugation of proteins and peptides continues to expand the application space of peptides and proteins with its precision, flexibility, and efficiency. It shows great potential to drive the development of emerging biotechnologies.
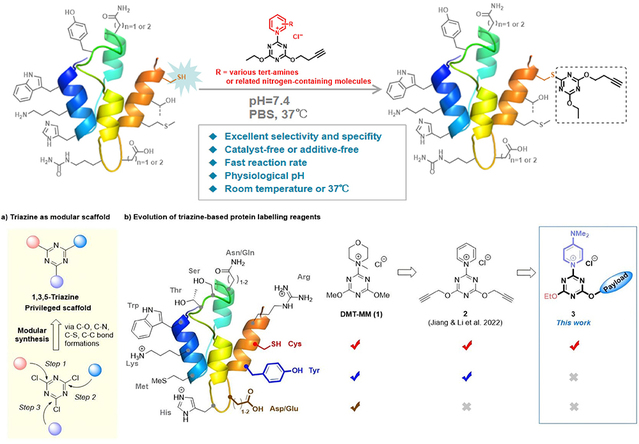
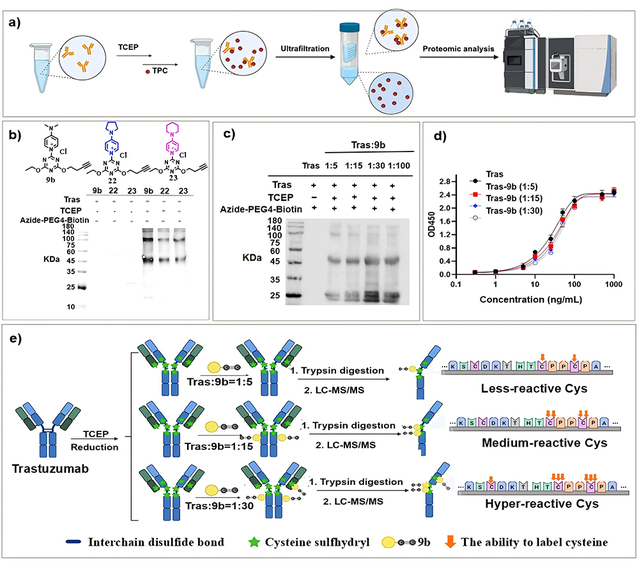
In 2022, the research team first reported triazine-pyridinium chemistry (TPC) probes capable of selective tyrosine bioconjugation, achieving proteome-wide, site-specific labeling of tyrosine residues (Chem. Commun. 2022, 58, 7066). In this study, the team developed a reagent that enables selective bioconjugation of cysteine (Cys) residues in peptides and proteins under physiological conditions. Mechanistic studies revealed that the introduction of a para-N,N-dimethylaminopyridinium group into the probe is crucial for achieving this high selectivity and that the C-N bond dissociation energy correlates positively with Cys selectivity, thus providing a theoretical basis for rational design. The optimized reagent 9b was successfully applied to Cys-selective labeling of trastuzumab, specifically modifying interchain disulfide bonds under reducing conditions while preserving antibody activity. This approach supports the construction of multi-site, high-drug-loading ADCs, and the resulting conjugates display markedly improved stability in human plasma compared with those prepared by traditional methods. It offers a new strategy for developing next-generation stable and controllable bioconjugate therapeutics.
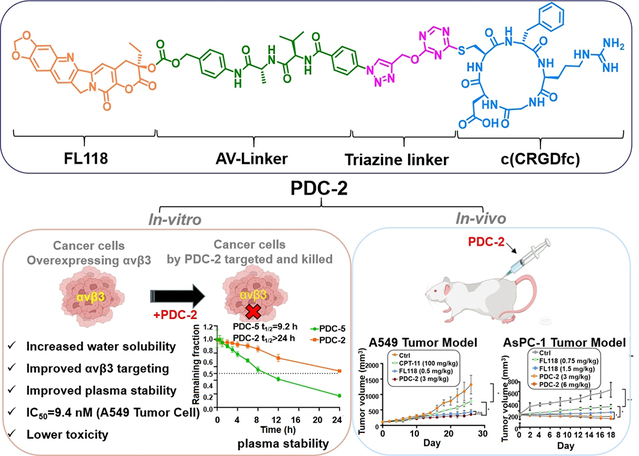
The team also successfully applied the triazine-based bioconjugation strategy to the development of PDCs. The related work, entitled “Conjugating 10,11-Dimethoxy-camptothecin with an Integrin αvβ3-Targeting Peptide through a Triazine Linker for Targeted Tumor Treatment in Lung and Pancreatic Carcinoma”, was published in Journal of Medicinal Chemistry. A key challenge of PDCs is their instability in the internal circulatory system, which can lead to payload release and systemic toxicity. By introducing a triazine linker, the team designed and synthesized the peptide-drug conjugate PDC-2, which shows high stability in plasma and achieves efficient tumor targeting and potent antitumor activity via integrin αvβ3-mediated internalization. Mechanistic studies revealed that PDC-2 dually inhibits Survivin protein expression and the PI3K/AKT/mTOR signaling pathway. In A549 and AsPC-1 xenograft models, PDC-2 demonstrated superior tumor growth inhibition, reduced systemic toxicity, and enhanced tumor specificity. Pharmacokinetically, it enables a sustained release of the parent drug, extending its half-life by 3.4-fold and promoting targeted tumor accumulation in tumor tissues, positioning it as a promising therapeutic candidate for lung and pancreatic cancers.