On December 13, 2025, the team led by Professor Dong Bo at the Fang Zongxi Center for Marine Evo-Devo, Ocean University of China (OUC), published their latest findings in Advanced Science. Their article, entitled “Halorotetin B, a novel terpenoid compound derived from marine ascidian, suppresses tumor growth by targeting the cell cycle regulator UBE2C”, reports a previously unreported terpenoid compound that suppresses tumor growth by targeting the cell-cycle regulator UBE2C. This discovery provides an important scientific basis for developing ascidian-derived antitumor drugs.
Cancer, characterized by high incidence and mortality, poses a serious threat to human health. Currently, the development of potent small-molecule chemotherapeutic drugs with reduced side effects is an effective strategy for cancer therapy. Natural products have long been recognized as a valuable source in antitumor drug discovery, owing to their structural novelty and chemical diversity. Marine ascidian (Halocynthia roretzi) is an edible ascidian rich in nutrients. Preliminary research has shown that ascidians harbor abundant symbiotic microorganisms, endowing them with the potential to produce diverse secondary metabolites. To date, only a few ascidian-derived bioactive natural products have been reported. Therefore, by using ascidians as the starting material, the project team identified natural products with antitumor activity, providing a theoretical basis for the development of antitumor drugs and their lead compounds.
In this study, researchers used cytotoxicity-guided screening to isolate active components that inhibit tumor cell proliferation. Using classical natural products chemistry, the team extracted, separated, purified, and structurally characterized the ascidian-derived natural products. They ultimately identified Halorotetin B, a novel terpenoid with potent antitumor activity. In vitro assays indicated this compound significantly inhibits the proliferation of multiple tumor cell lines while exhibiting low toxicity toward normal cells. In vivo pharmacodynamic studies further demonstrated that it markedly suppresses tumor growth. Mechanistic studies revealed that Halorotetin B influences cell-cycle signaling in tumor cells. Chemical proteomics combined with transcriptomic analyses identified the cell-cycle regulator UBE2C as a direct molecular target of Halorotetin B. By directly binding to UBE2C, Halorotetin B inhibits the ubiquitin-mediated degradation of the downstream cell-cycle regulators, cyclin B1 and securin. Thereby, it induces robust tumor cell cycle arrest, promotes tumor cell senescence, and ultimately inhibits tumor proliferation.
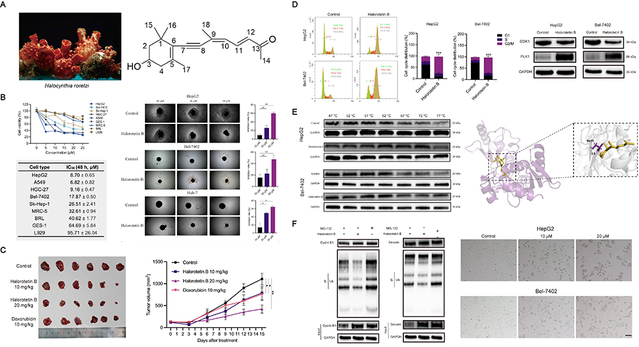
Successful experience in tumor-targeted therapy has shown that the discovery of new therapeutic targets is crucial for the development of promising antitumor drugs. Recent studies indicated that targeting the ubiquitin-proteasome system represents a potential strategy for antitumor drug discovery. Several small-molecule inhibitors targeting the ubiquitin-proteasome system have been reported, including the allosteric inhibitor CC0651, which targets Cdc34, and DHPO, which targets UbcH5c. As an E2 ubiquitin-conjugating enzyme, UBE2C is highly expressed in many types of tumors and is associated with tumor cell proliferation, metastasis, and resistance to chemotherapeutic drugs. UBE2C is a promising target for controlling tumor progression. To date, no canonical, direct, and potent small-molecule UBE2C inhibitor has been reported. Therefore, as a novel small-molecule UBE2C inhibitor, Halorotetin B holds strong potential as a valuable lead compound for the development of new anti-tumor drugs.








