Recently, the research team led by Professor Wang Yang from the School of Medicine and Pharmacy at Ocean University of China (OUC) has made new progress in the asymmetric catalytic construction of chiral nitrogen heterocycles. The article entitled “Asymmetric Catalytic Construction of 1,1-Diheteroquaternary Tetrahydro-β-Carbolines Enables Collective Syntheses of Polycyclic Alkaloids” was published in the Journal of the American Chemical Society.
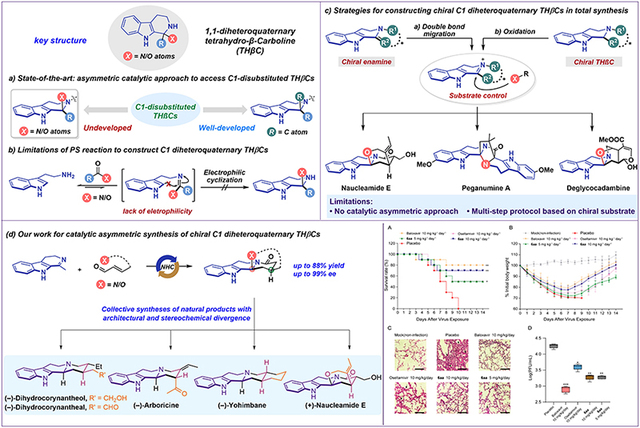
Between 2013 and 2023, 64% of new small-molecule drugs approved by the FDA were chiral, and 82% contained nitrogen heterocycles, underscoring the growing importance of chiral nitrogen-containing heterocyclic scaffolds in drug discovery. However, the asymmetric synthesis of chiral nitrogen heterocycles remains highly challenging. In particular, the 1,1-disubstituted tetrahydro-β-carboline (THβC) scaffold is a privileged framework with distinctive structural features and diverse biological activities. 1,1-Disubstituted THβCs bearing a C1 diheteroatom-bearing quaternary carbon center are prevalent in natural products and pharmaceutical molecules, yet catalytic asymmetric approaches to this scaffold remain elusive. Traditional methods, such as the Pictet–Spengler reaction, are generally ineffective for heteroatom-substituted carbonyl substrates, which hampers the key cyclization step and thus the asymmetric construction of such structures. In this context, the research team has developed an asymmetric catalytic annulation between dihydro-β-carbolines and enals to construct chiral C1 diheteroquaternary THβCs, enabling concise collective syntheses of multiple complex indole alkaloids. This approach also reveals excellent anti-influenza virus activity by targeting the viral NS1 protein.
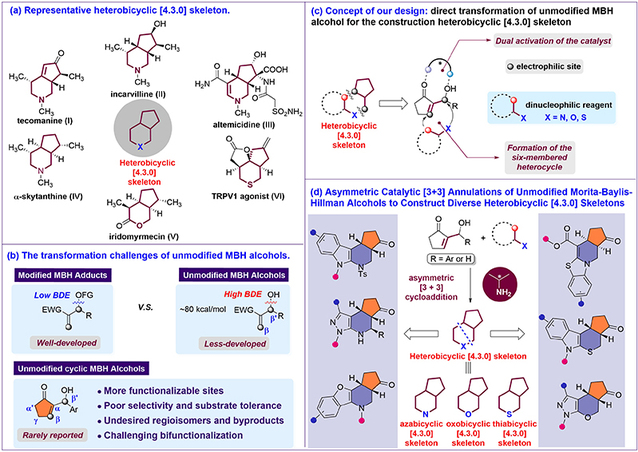
Heterobicyclic [4.3.0] skeletons, including azabicyclic [4.3.0], oxobicyclic [4.3.0], and thiabicyclic [4.3.0] skeletons, are widely found in natural products and pharmaceutically important molecules. In existing synthetic strategies toward heterobicyclic [4.3.0] skeletons, chiral centers are typically introduced at an early stage, and multiple transformation steps are required. Recently, the research team reported their latest progress in this field in a paper entitled “Asymmetric Catalytic [3+3] Annulations of Unmodified Morita-Baylis-Hillman Alcohols to Construct Diverse Heterobicyclic [4.3.0] Skeletons”, published in the internationally renowned journal JACS Au. Using a chiral primary amine-acid synergistic dual-activation strategy, the team successfully activated unmodified MBH alcohols and developed a β,β′-site-selective asymmetric [3+3] annulation of these substrates. This general platform enables the construction of a series of structurally diverse, highly enantioselective chiral heterobicyclic [4.3.0] skeletons, including aza-, oxo-, and thia-bicyclic [4.3.0] frameworks, featuring a broad substrate scope, excellent stereocontrol, and good compatibility with late-stage modification of drug-like molecules. The reaction proceeds under mild conditions and is applicable to a variety of bioactive molecules, allowing convenient synthesis of numerous drug analogues and providing a general and efficient solution for MBH chemistry, the synthesis of chiral heterobicycles, and the rapid preparation of bioactive molecules.








